Summary of Multicenter Open Labeled Clinical Study in Advanced Breast Cancer Patient
By: Dr. Med. Harald Merckens
Original title: Mistelstudie bei Mammakarzinom in Ägypten. Der Merkurstab 2003;56(2):99-100.
Article-ID: https://www.anthromedics.org/DMS-18266-DE
This translation is published with the kind permission of the journal Der Merkurstab.
In an open multicenter study(1) involving 9 oncological centers in Egypt, the effect of Viscum fraxini 2®(2) was investigated in 26 breast cancer patients with advanced illness. The study was conducted by Mahfouz et al. in collaboration with H. Werner, A. Scheffler of the Carl Gustav Carus Institut and I. Abouleuísh of Atos Pharmaceuticals (Sekem), Cairo.
Of the 26 patients, 11 were premenopausal and 15 were postmenopausal. 11 already had metastases (liver, lung, bone, lymph node, skin). Criteria for inclusion in the study were a Karnovsky Index > 70% and a life expectancy > 6 months. All conventional therapy options (surgery, radiotherapy, chemotherapy, hormone therapy) had been exhausted. One patient had not been previously treated.
In the treatment procedure, 3 ml of Viscum fraxini 2 were injected peritumorally, intratumorally or both, 1 x weekly. No accompanying medication was given; only paracetamol at low dose was offered in cases when mistletoe fever exceeded the patient’s subjective tolerance level. The observation period lasted 16 weeks; 18 patients continued treatment for a further period of up to 136 weeks.
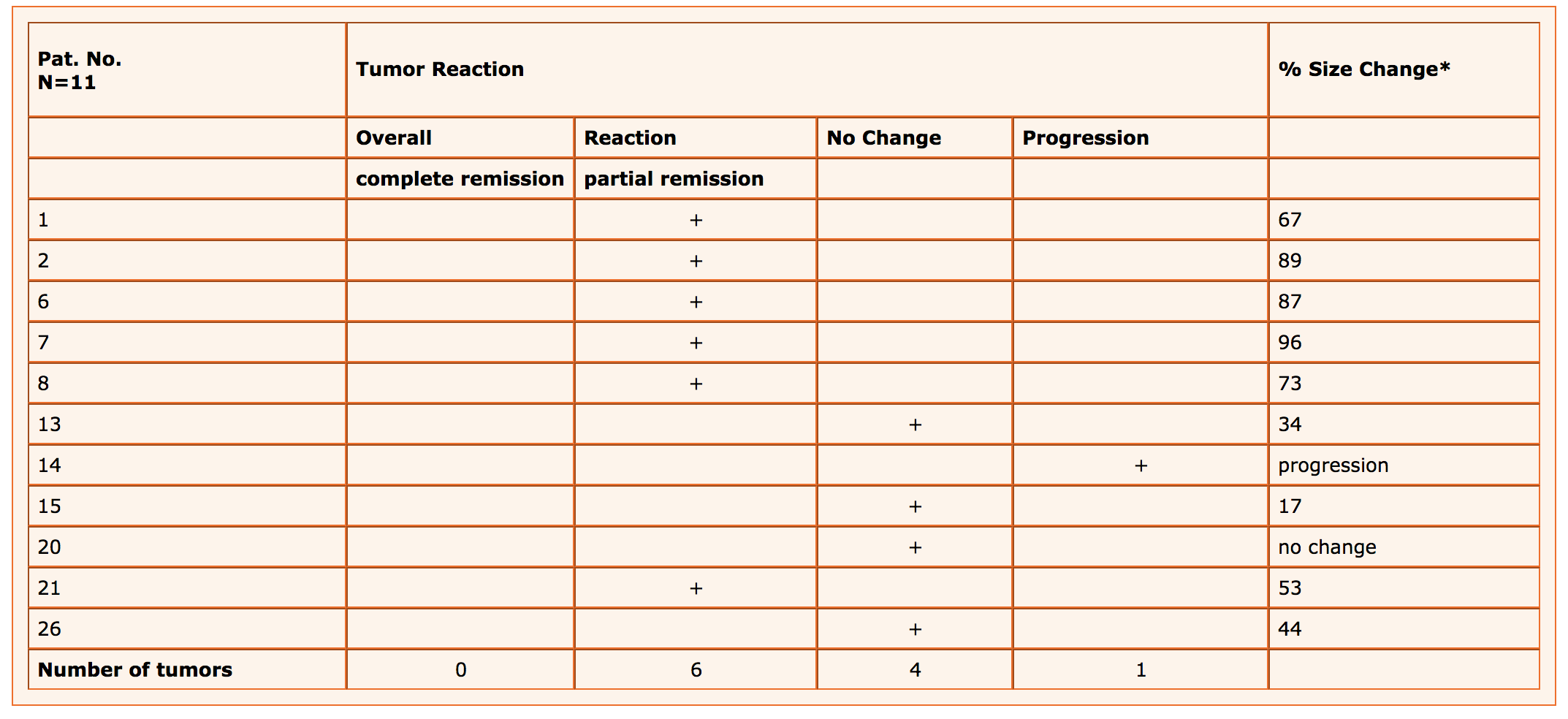
Of the premenopausal patients, 6 responded to the therapy with a 17 – 90% decrease in tumor size. In 4 patients the tumor size remained unchanged, while in one patient it showed progression (tab. 2).
Of the 15 postmenopausal patients, 10 (or 2/3) responded with a decrease in tumor size, 2 of them with complete remission (!), while in 5 patients the tumor remained unchanged. There were no cases of progression (tab. 3). 18 patients from both groups continued the therapy beyond the period of the study; 14 of the patients showed a response, while in 4 the tumor remained unchanged.
The authors conclude that there is no obvious difference in responsiveness to viscum therapy among pre- and postmenopausal patients. A further finding was that continuation of the therapy beyond the 16-week period covered by the study is effective. In this group 11 of the 18 patients already had metastases, which showed a good regression response to this therapy (tab. 4).
This study reveals excellent results for mistletoe treatment of patients with advanced breast cancer. Two particular considerations should be added: First, the treatment was conducted using a single high dose of mistletoe once each week, which typically evokes fever reactions at least towards the beginning of the treatment. The fever reactions appear to have been crucial to the efficacy of the treatment in this study—and it is in fact a principle in the approach to tumor treatment advocated by Rudolf Steiner. The single weekly dose is also a significant factor in its efficacy. Secondly, the fact that the patients were Egyptian women also plays a role, as they display a better responsiveness to this form of therapy than is commonly experienced here in Germany. Thanks are due to the authors for their work on this study, which impressively documents the efficacy of mistletoe therapy.
Dr. med. Harald Merckens
Medical Director
Paracelsus-Krankenhaus
Burghaldenweg 60
D-75378 Bad Liebenzell
Table 1(3) : Reaction of primary breast lesions in 11 premenopausal breast cancer patients treated with Viscum fraxini 2® for 16 weeks
* Based on tumor size prior to Viscum treatment and in the 16th week
Table 2(4) : Reaction of primary lesions in the breast in 15 postmenopausal breast cancer patients treated with Viscum fraxini 2® for 16 weeks
* Based on tumor size prior to Viscum treatment and in the 16th week
Table 3(5) : Reaction of primary lesions in the breast in 18 postmenopausal breast cancer patients treated with Viscum Fraxini®2 for more than 16 weeks (18 – 136 weeks)
Table 4: Reaction of the metastases in 11 breast cancer patients treated with Viscum Fraxini®2 for more than 16 weeks (18-136 weeks)
Literature and Notes
Mahmoud Mahfouz, M.D. et al.: Multicenter open labeled Clinical Study in Advanced Breast Cancer Patients. A preliminary Report. Journal of the Egyptian Nat Cancer Institute, Vol. 11, No.3.September: 221-227,1999
Corresponding to Abnoba viscum fraxini 2®
Table 1: 6 of 11 patients—over half of the group—showed good responsivenesss in the sense of a partial remission.
Table 2: 2 patients manifested a complete remission (!) and 8 a partial remission. Thus, a positive response occurred in 2/3 of the cases.
Table 3: In protracted treatment—longer than 16 weeks—the response rate with partial or complete remission comes close to 4/5 of the cases.
Citation: 2004 English edition? zz